Lanomax®
The 'lanosterol eye drops' that claim to dissolve cataracts have appeared.
A study released in July 2015 shows that lanosterol, a natural chemical produced in our bodies, can effectively uncloud cataracts. To date, there have been no medicines known to cure cataracts, and as such, a surgical procedure to remove the opacified lens and replace it with an artificial lens has been the only available option to correct the problem. However, if cataracts could be cured with simple eye drops such as ‘lanosterol eye drops,’ that would greatly reduce the need for cataract surgery to treat the condition. Therefore, there has been a great deal of interest in the findings of the study in the ophthalmological field worldwide.
Dr. Kang Zhang, Professor of Ophthalmology and Chief of Ophthalmic Genetics at UC San Diego, and his research team have found that the organic compound called lanosterol can dissolve protein blocks that cause cataracts and destroy lens opacity. They published their findings in Nature magazine on July 22, 2015.
Their work began with the cases of three children who had a severe cataract condition that ran in the children’s family. The scientists sequenced the children’s genomes and identified a genetic mutation that interfered with the production of lanosterol, a naturally occurring steroid in the body. In addition, the researchers found that lanosterol is used in the body to synthesize cholesterol and several steroid hormones, but the crystalline lens of the eye also contains an abundance of this substance. That clue led them to the decision to test whether lanosterol might have the ability to prevent or even eliminate cataracts.
They first removed the lenses of 13 cataracted rabbits and immersed the lenses in a 25 mM lanosterol solution followed by an incubation period of 6 days. At the end of the 6-day experiment, transparency was significantly improved in the rabbits’ lenses.

However, a lanosterol solution used only in the form of eye drops will not cure cataracts without the injection.
Immediately after Zhang’s research team published the paper, there was suspicion raised as to whether or not lanosterol in the form of eye drops alone can cure cataracts. Because lanosterol has extremely low solubility, it is not easy for the lanosterol molecules in eye drops to reach the crystalline lens. In fact, in the experiments on the seven dogs, Zhang’s team had to anesthetize all of dogs and inject the lanosterol solution directly into the vitreous body of the eyes in addition to administering the lanosterol eye drops.
A paper supporting this suspicion was published with a title of “Effect of lanosterol on human cataract nucleus” by C. K. Minija’s research team in December 2015, five months after the publication of the Zhang team’s Nature paper. The conclusion of Minija’s paper was that the lanosterol solution was not effective for the treatment of human cataractous nuclei when the laboratory test was performed using simulation experiments based on the Nature paper published in July 2015.
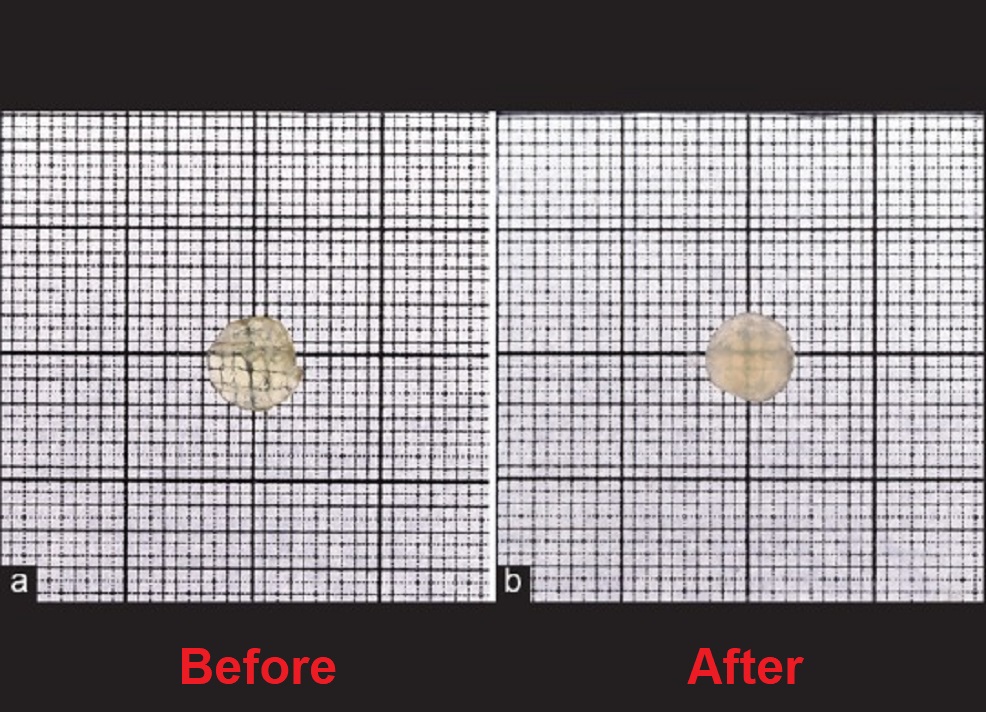
The team immersed the human lens nuclei extracted during cataract surgery in the same 25 mM lanosterol solution as used in the Zhang team’s research and incubated them for 6 days. At the end of the 6-day experiment, the transparency of the human lens nuclei immersed in the lanosterol solution was not improved at all.
Since the lens nucleus is free of the lens coat, the effect of lanosterol on cataract removal was expected to be much stronger. However, contrary to this expectation, the treatment effect of lanosterol in the rabbit and dog lenses as shown in Zhang’s research did not appear at all in the human lens. This means that the lanosterol solution used by Zhang’s and Minija’s teams could not deliver the lanosterol molecules it contained into the human lens.
This pessimal experimental result is due to the extremely low chemical solubility of lanosterol and its extremely low permeability within the body tissues. Even with liposomes, nanoparticles, or dextrin-based carriers, it is still difficult to overcome these limitations. Therefore, in a non-laboratory setting, it is practically impossible to dissolve cataracts only through the administration of lanosterol eye drops to animals or humans with cataracts. This is because the lanosterol molecules contained in the lanosterol eye drops must pass through additional barriers, such as the cornea, anterior, iris, and pupil, just to reach the outer surface of the crystalline lens alone, not to mention the inside of the lens.
It is clear what the experimental results of both Zhang’s and Minija’s teams mean; while lanosterol is certainly related directly to the treatment of cataracts, the simple lanosterol solution as used by both the research teams has a limitation in transferring the lanosterol molecules into the crystalline lens. Hence, simple lanosterol eye drops alone cannot deliver the lanosterol molecules into the lens, which is why Zhang’s team injected the lanosterol solution directly into the vitreous body of the eyes after anesthetizing the seven dogs. In conclusion, the simple lanosterol solution as used by Zhang’s and Minija’s teams cannot be expected to have any cataract treatment effect when used solely in the form of eye drops. The drops must be used in conjunction with injections.
Lanomax® can cure cataracts in the form of eye drops alone; no injections are necessary.
Lanomax® uses ThruDelivery®, an innovative drug delivery system built using the latest advanced nanotechnology, to deliver lanosterol molecules into the crystalline lens of the eye. This system separates the lanosterol molecules from the carriers in the carrier-lanosterol complex attached to the corneal surface, and then quickly passes them through the cornea, anterior, iris and pupil to deliver them into the crystalline lens. It is because of this ThruDelivery® system that Lanomax® is the only eye drop on the planet to show a real therapeutic effect on cataracts.
We discovered this ThruDelivery® system by fortuitous chance in the winter of 2015, and have decided not to disclose its secrets to the world by way of patents or articles. It’s like Coca-Cola’s manufacturing know-how. Currently, this amazing lanosterol delivery system is only found in Lanomax® eye drops. However, please be advised that we cannot inadvertently include information about this system in the ingredients table of Lanomax®.
A research paper has been published that Lanomax® has a therapeutic effect on human cataracts.
Paper title: Lanomax as a Drug in Cataract Treatment: A Case Study
Abstract: The possibility of determining lanosterol, the active ingredient of the drug Lanomax, in the lacrimal fluid by a new electrochemical method of multisensory stripping voltammetry was studied. The measurements were carried out using a planar solid-state electrode. The test-system solution was a 0.05 M KCl solution, which contained the Zn2+, Cd2+, Pb2+, Co2+ and Hg2+ metal cations at the concentration of 5*10-5 M. It was shown that the method is effective for the determination of lanosterol; the dynamics of the change in its concentration in time was considered. It was also shown that the lanosterol concentration in the lacrimal fluid remains constant for 12 h. The possibility of nonsurgical dissolution of cataracts in humans using Lanomax was investigated. In the course of the dynamic observation of this process for 8 months, the stabilization of rapidly progressive cataract was found.
Lanomax® Drug Facts
|
